Attention
This website is best viewed in portrait mode.
-
industries
- healthcare and life sciences
- medical device regulatory compliance
-
Post-Mergers & Acquisitions (M&A) Integration Services
Post-Mergers & Acquisitions (M&A) Integration Services
Harmonise | Comply | Sustain

Post-Mergers & Acquisitions (M&A) Integration Services
Harmonise | Comply | Sustain
Enabling Seamless Mergers and Acquisitions through Compliance-Driven Services
In the dynamic landscape of MedTech M&As, the need for seamless integration has intensified. From cross-border deals to acquiring digital health innovations, embracing vertical integration, and consolidating in niche markets, many factors drive M&A integration in the medical device industry.
The challenges such as integration complexities, regulatory hurdles, financial risks, and soaring integration costs, highlight the importance of adept Quality Assurance and Regulatory Affairs (QARA) as a part of M&A integration services.
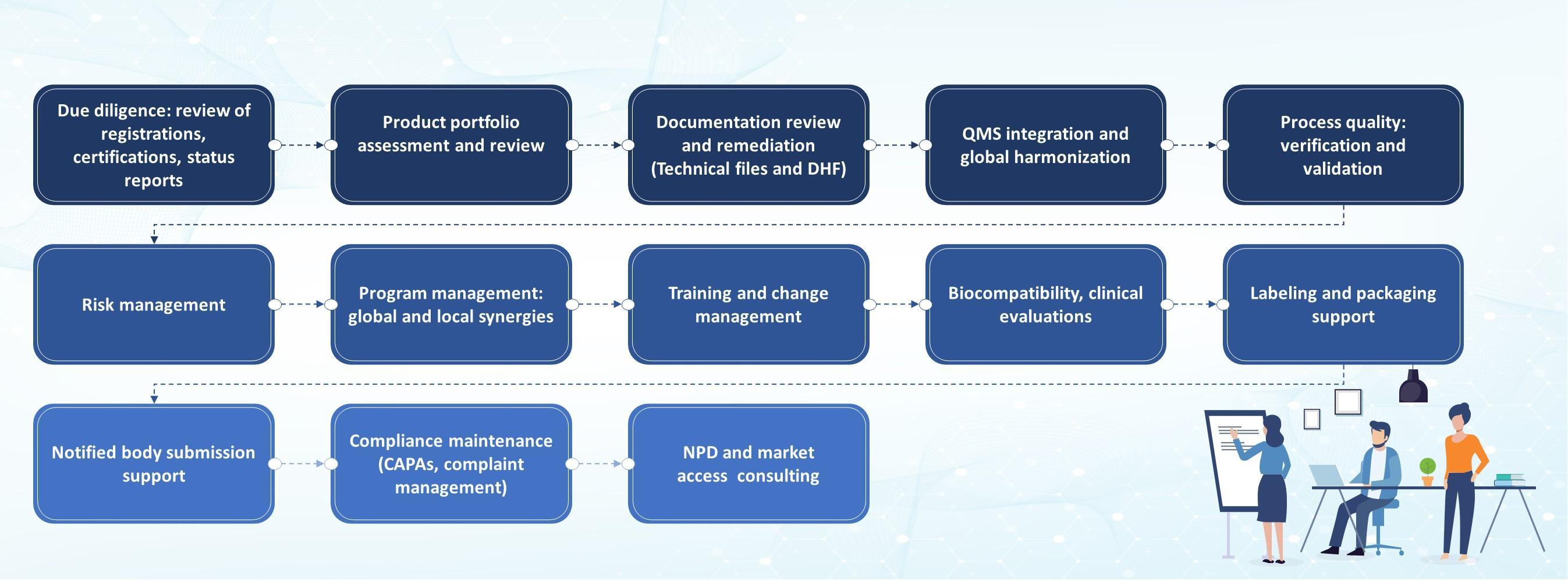
Tata Elxsi is your strategic partner in navigating the complexities of MedTech M&A integration. Our merger integration solutions include post-acquisition due diligence, facilitating a seamless transition, QARA solutions like QMS integration, DHF creation and remediation, and risk management. We also provide support for labeling, IFU, drawing, and packaging. With expertise in manufacturing documents, biocompatibility, clinical compliance, and regulatory submissions, Tata Elxsi ensures a seamless M&A integration process that provides a resilient foundation for MedTech companies to thrive.

Here's how we can help you

Comprehensive regulatory due diligence for acquired product portfolios
- Assess compliance history to identify regulatory risks and liabilities
Integration of products and quality processes for compliance
- Proactively address evolving compliance requirements to mitigate risks
Streamline operational efficiencies post-acquisition
- Implement product and quality change management processes for sustained compliance
- Optimise product portfolio documentation by creating product families and groups
M&A Integration Service Framework
Why Tata Elxsi?
- Collaborate with M&A integration planning teams during the due diligence phase to define objectives and achieve the plan goals
- Catalogue pricing and an outcome-based commercial model to support customers through the unpredictable variability and volume of activities
- Compliance assurance model with a track record of transformative productivity gains
- Certified engineers, regulatory and quality experts along with comprehensive in-house training modules to ensure fast ramp-up
- Data security in compliance with ISO 27001:2013, ISO 13485 QMS certification, 21 CFR Part 820
Subscribe
To subscribe to the latest updates & newsletter