Attention
This website is best viewed in portrait mode.
-
industries
- healthcare and life sciences
- medical device regulatory compliance
-
Clinical Evaluation Report Consulting & Writing
Clinical Evaluation Report Consulting & Writing
Assess | Analyze | Execute

Clinical Evaluation Report Consulting & Writing
Assess | Analyze | Execute
Enhancing Clinical Efficacy and Safety

The introduction of EU MDR/IVDR has led to rigorous post-market oversight and re-classification of devices. Comprehensive clinical and performance evaluation processes across medical device classes are thus crucial to ensure conformance to performance and safety norms.
This requires preparation of clinical and performance evaluation packages - literature reviews, data gathering, analysis and interpretation, risk-benefit assessment and re-assessment of claims. Many manufacturers lack clarity of the best practices for creating such Clinical and Performance Evaluation Reports (CER/PER).
Tata Elxsi's diverse team of medical writers, clinical evaluation consultants, system engineers, and physicians/surgeons (MD) have vast experience in MEDDEV 2.7.1/Rev 4, EU MDR 2017/745, and IVDR 2017/746. We can be your perfect partner as you embark on this journey, providing the expertise needed to navigate regulatory requirements and innovate seamlessly.


Here’s How We Can Help You

Compliance roadmap creation with strict adherence to regulatory requirements
- Ready-to-use customisable compliance checklists and templates
- Global regulatory intelligence platform, TEDREG, available for alerts to adapt to everchanging industry legislations and standards
Greater clinical evidence for medical and IVD devices to boost market acceptance
- Streamlined data optimisation
- Quicker response to changes in major geo-regulatory requirements preventing high-volume rework
Faster time-to-market
- Proactive engagement with Notified Bodies to minimise operational risks
- Efficient maintenance and retrieval of records for sustained compliance
Service Framework
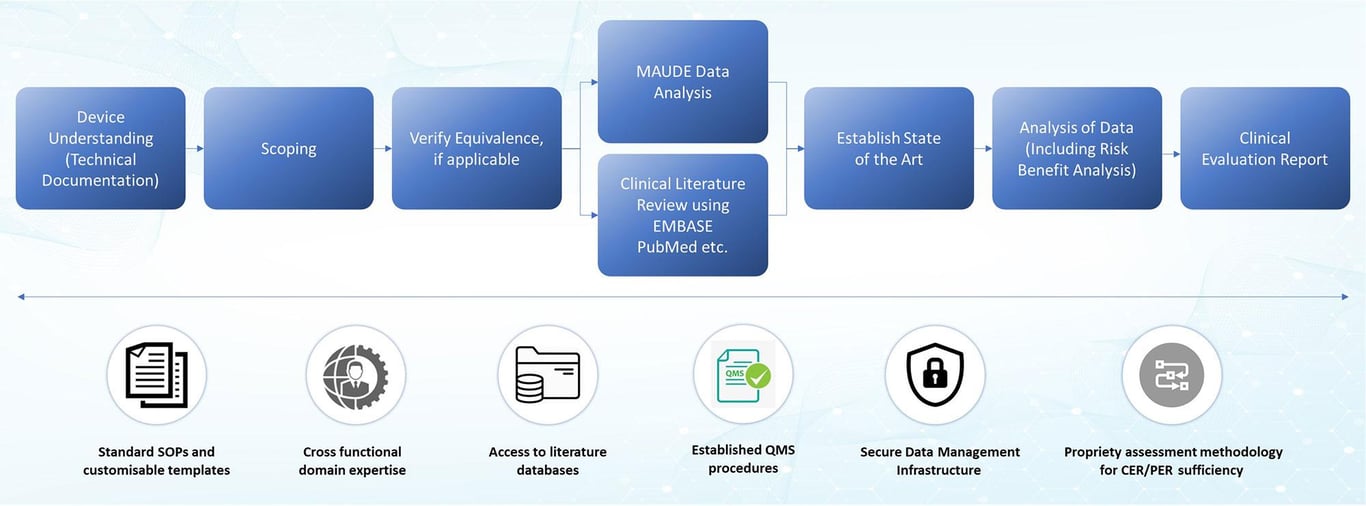
Why Tata Elxsi?
Expert Compliance and Regulatory Team with over a decade experience
- Assistance for scientific communication
- ICH-GCP certified clinical research experts to ensure timely delivery of Clinical Data Management (CDM)
Tata Elxsi's TEDREG: Global Regulatory Intelligence Platform
- Rule-based automated daily update alerts and notifications to stay informed about the latest regulatory changes
- Dashboards, bookmarks, and workflow management to enhance operational efficiency
- Tracking specific events with search strings to retreive precise regulatory information
Ensure uninterrupted sale of devices
- Largest global offshore team of biomedical experts for rapid training and guidance to accelerate product development
- Timely management of updated documents for submission to notified bodies to streamline the regulatory approval process
Subscribe
To subscribe to the latest updates & newsletter