Charting the Future of Radiology: Innovation, Regulation, and Real-World Impact
Distinguish genuine innovation from hype, operationalize compliance for SaMD and AI/ML-enabled devices, and harness real‑world data (RWD) and post‑market evidence (RWE) to continuously improve safety, performance, and trust.
Watch Now

Beyond EU MDR: Innovate and Ensure Patient Safety
Discover how medical device makers can go beyond compliance to drive innovation and patient safety under the evolving EU MDR landscape.
Read ArticleThe Vital Role of QARA in the Medical Device Industry
In the dynamic landscape of the medical device industry, Quality Assurance and Regulatory Affairs (QARA) stand as the guiding pillars that ensure the highest standards of product quality and regulatory compliance. Spanning across the entire life cycle of medical devices, QARA plays a pivotal role in upholding the safety, efficacy, and reliability of the devices. QARA is the cornerstone of product quality, encompassing a comprehensive range of processes and practices aimed at upholding stringent industry standards and regulatory requirements.
It encompasses the earliest stages of product design and development, spanning through manufacturing, distribution, and post-market surveillance. By meticulously aligning each phase with established norms, QARA safeguards against potential risks and uncertainties, fostering a culture of excellence and integrity within the industry.
Driving QARA Programs through a Global Engineering Network


What we do
Tata Elxsi offers comprehensive Quality Assurance and Regulatory Affairs (QARA) services to support medical device companies throughout their product lifecycle. Our experienced team ensures strict adherence to global regulatory standards, enabling efficient approvals for market entry. We assist in developing and implementing robust Quality Management Systems (QMS) tailored to individual client needs, ensuring consistent product quality and compliance. With expertise in risk management, we identify potential hazards and apply effective mitigation strategies to enhance product safety. Our QARA specialists provide meticulous support during audits, both internal and external, ensuring clients meet regulatory requirements. We streamline regulatory submissions, facilitating faster approvals from authorities like the FDA and EMA. Post-market surveillance is a priority, as we monitor product performance and handle adverse event reporting diligently. Trust Tata Elxsi for QARA solutions that prioritize patient safety, regulatory compliance, and successful market access.
Solution Framework
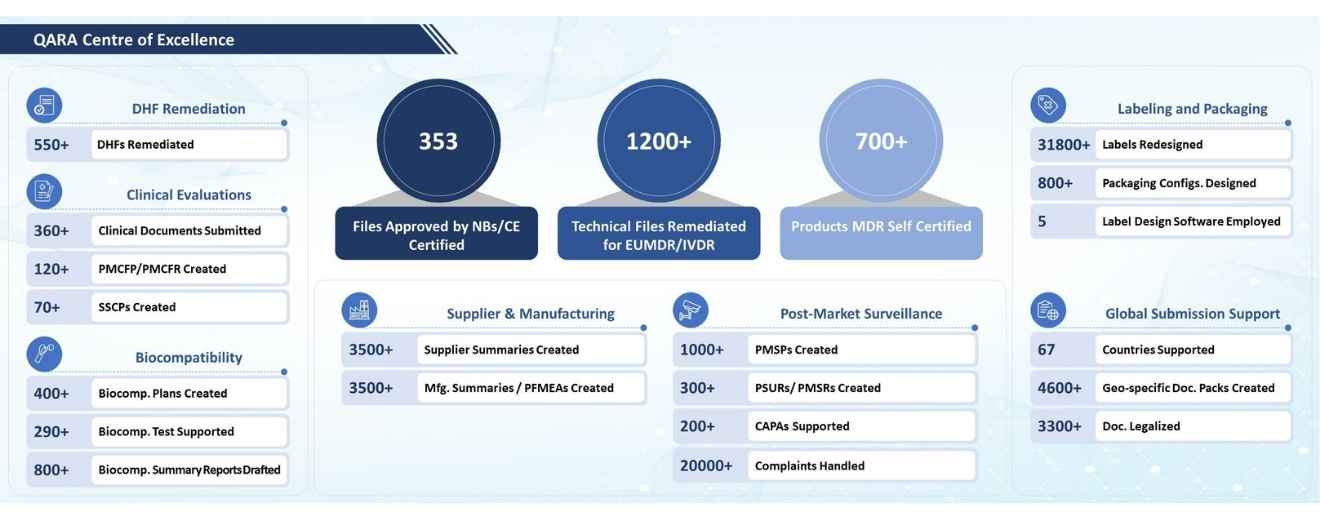
Quality Management and Assurance
- QMS Remediation (ISO 13485, Regulatory Guidelines)
- DHF Remediation
- CAPA Handling
- Complaints Management
- Audit Support
- Non-Conformities and Deficiencies Assessment and Addressal
Regulatory Affairs and Compliance
- EU MDR/IVDR Compliance
- FDA 510K, De-Novo, PMA
- Post-submission Support (NBs Draft Responses)
- Global Submissions
- Biocompatibility
- Labelling & Packaging
- Manufacturing and Supplier Support
- Regulatory Strategy and Pathway Planning
- Regulatory Intelligence
- Clinical Evaluations and Post-market Surveillance
Risk Management
- Risk Management Report (ISO 14971)
- DFMEA, PFMEA Creation/Update
- Risk Mitigation
- Hazardous Substance (PFAS, ROHS, REACH, Prop-65)
- Risk-Benefit Analysis
Why Tata Elxsi?
-
Catalog pricing and outcome-based commercial model to support customers through the unpredictable variability and volume of activities.
-
Pool of readily deployable certified regulatory experts, product engineers and doctors.
-
Proprietary methodology to help stakeholders appropriately plan for transition.
-
Centralized program management to foster cross-functional collaboration.
In Focus
Information Hub
-
Why is QARA crucial in the medical device industry?
QARA ensures that medical devices are safe, effective, and compliant with global regulatory standards. It spans the product lifecycle—from design controls and risk management to audits and post-market surveillance. With rising regulatory scrutiny, QARA minimizes market entry delays and mitigates compliance risks, helping manufacturers maintain product quality and patient safety.
-
What are the key trends shaping QARA today?
Global harmonization, EU MDR/IVDR transitions, real-world evidence integration, and AI/ML regulation are key drivers. Regulatory bodies are emphasizing risk-based approaches, digital traceability, and sustainability (PFAS, RoHS). Companies must evolve their QARA practices to remain agile and future-ready.
-
How can QARA help accelerate product approvals?
By ensuring proper documentation, audit readiness, and proactive compliance strategies, QARA streamlines the regulatory submission process (e.g., 510(k), CE marking). Strong QARA practices reduce rework, improve reviewer confidence, and facilitate faster regulatory approvals across markets.
-
What role does risk management play in QARA?
Risk management is central to QARA. It involves identifying hazards, estimating risks, and implementing controls per ISO 14971. Tools like FMEA (DFMEA, PFMEA) help manufacturers preempt failures and ensure regulatory compliance, enhancing product safety and lifecycle traceability.
-
Can QARA be outsourced, and what are the benefits?
Yes. Outsourcing QARA to experts like Tata Elxsi gives access to specialized talent, global regulatory intelligence, and scalable processes. It reduces operational burden, enhances submission quality, and supports faster time-to-market—especially for startups and mid-sized manufacturers.













