Blog
Worst-Case Assessments to Best-Case Solutions: Optimizing Biocompatibility Testing for Medical Devices
Introduction
Ensuring the biological safety of medical devices is a fundamental aspect of regulatory compliance and patient safety. Biocompatibility testing serves as a critical method to verify that devices are safe for use; however, comprehensive testing of every individual device can impose significant resource burdens, including increased manpower, animal use, testing costs, non-availability of test lab slots and potential delays in regulatory approval processes. To address these challenges, the worst-case approach offers a strategic solution that enhances testing efficiency while maintaining safety standards.
 Critical Criteria for Worst-Case Assessment
Critical Criteria for Worst-Case Assessment
Overview of the Worst-Case Approach
The worst-case approach involves identifying and selecting the device with the highest biological risk within a product family or category for testing purposes. By demonstrating safety for the representative, most challenging device, manufacturers can infer the safety of other devices with lower risk profiles by leveraging the testing results obtained from high-risk device to the lower risk devices. This approach also aligns with the principles of the 3Rs—Replacement, Reduction, and Refinement—in animal testing, promoting animal welfare and ethical research practices. While many OEMs worldwide commonly adopt the worst-case approach for various testing procedures, such as cleaning, sterilization, packaging, and shelf-life validation, applying this approach to biocompatibility testing necessitates a different viewpoint and the consideration of varied strategies.
Importance of Worst-Case Scenarios in Biocompatibility
The primary goal of employing a worst-case approach is to facilitate robust testing, thereby ensuring patient safety and regulatory compliance. This methodology also enhances resource optimization, reduces testing burdens, and accelerates the regulatory approval process. Additionally, it supports continuous adherence to evolving regulatory standards and aligns with risk management frameworks in accordance with ISO 14971.
How Worst-Case Assessment Approach helps within Product Families?
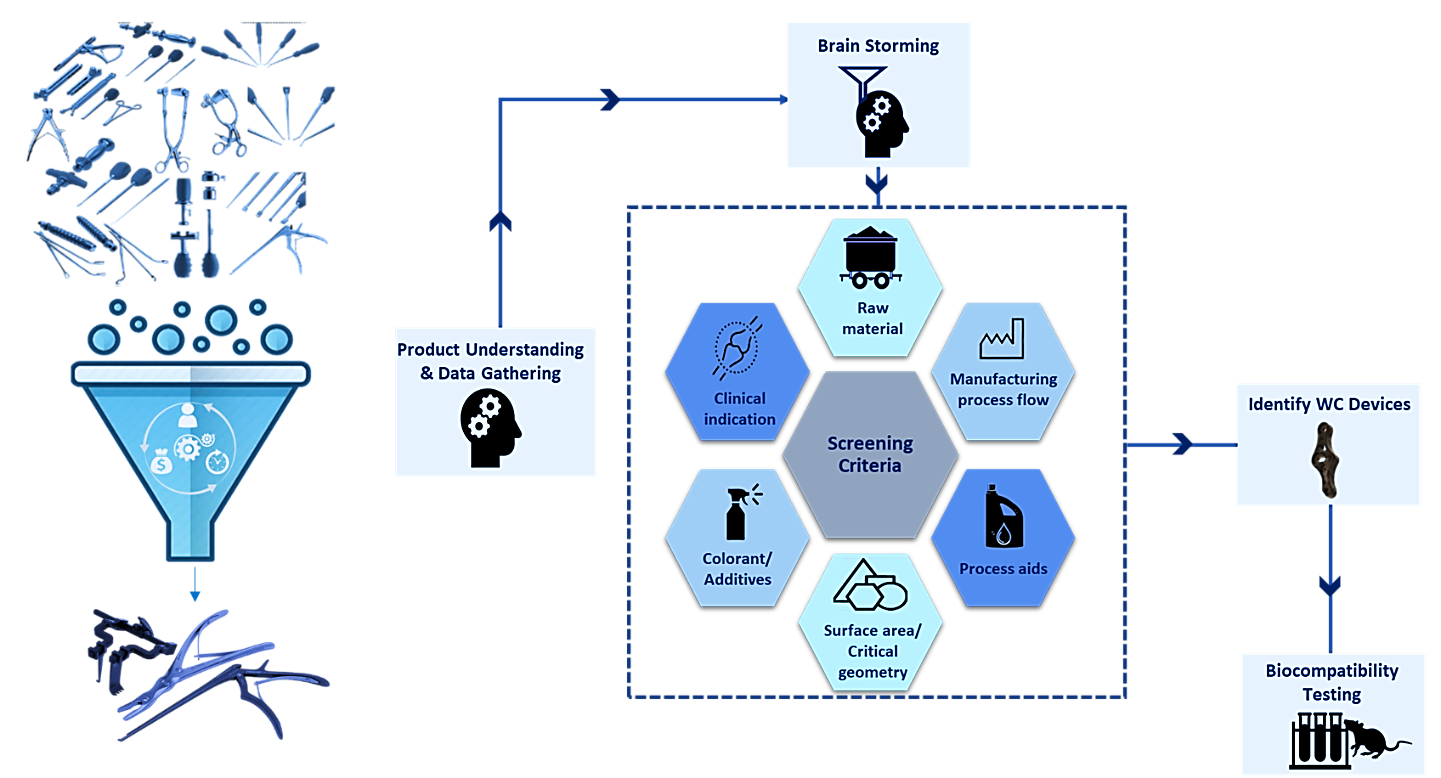
Medical devices are often produced in families comprising multiple components or variations sharing similar features and functions. Testing every component individually is neither practical nor recommended. Instead, selecting representative samples from each device family for testing—particularly those embodying the worst-case characteristics—enables efficient risk assessment. Accurate identification of the worst-case device necessitates comprehensive understanding of design complexity, materials, manufacturing processes, intended use, etc.
Determining the worst-case device within a family of devices, such as implantable devices with different dimensional variants, is usually straightforward. It typically involves selecting the implant with the largest dimensions or the greatest surface area exposed to the patient. However, this task becomes much more complex when the device family includes multiple instrument types with diverse designs and intended uses. Moreover, numerous additional factors can introduce further variability and complexity into these evaluations.
Developing a scientifically robust strategy for leveraging data across multiple product families requires a collaborative brainstorming session with a diverse skill set. Gathering accurate and comprehensive data—often from suppliers and internal sources—is essential. In cases of limited empirical data, extrapolation and scientific judgment become necessary to support decision-making. Document each step and method used to identify the worst-case device(s), providing clear rationale and robust data to support the conclusions. This ensures the assessment is thorough, transparent, and reliable.
Regulatory Developments and Implications
Recent revisions to ISO 10993-1 underscore a heightened focus on testing requirements, especially concerning genotoxicity assessments. For devices with prolonged or systemic contact, genotoxicity testing has become mandatory (read more on the Impact on Genotoxicity). These regulatory updates may pose additional challenges, particularly for legacy products, but adopting a worst-case strategy allows manufacturers to leverage data efficiently across multiple product lines. By selecting the most challenging device within a family—characterized by the highest complexity, material diversity, or manufacturing variability—companies can demonstrate safety comprehensively. In addition, ISO 10993-1 revision put emphasis on reassessing the testing strategy to ensure they cover lifecycle impacts, including cumulative and prolonged exposures (read more on Device Categorization). These changes will likely necessitate additional testing, especially for devices that were previously exempt from rigorous evaluations. Here, the manufacture can pick worst-case device that represents the most challenging conditions for testing within a product family.
The concept of a medical device family is likely to be included in the final version of ISO 10993-1, particularly in the context of selecting a representative worst-case device for finished device testing. Additionally, the device manufacturer must document the rationale behind choosing the worst-case device, emphasizing the critical importance of this decision. This rationale should also be included in the Biological Evaluation Plan.
Benefits and Continuous Monitoring
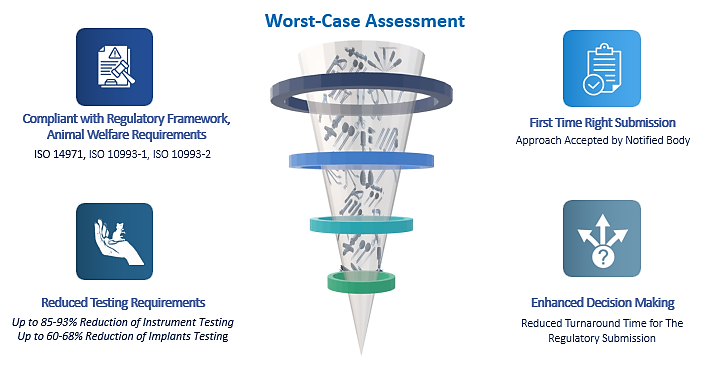
Adopting a comprehensive worst-case approach offers a strong basis for biocompatibility evaluations, streamlining regulatory submissions and minimizing redundant testing. Additionally, optimizing this process can be beneficial, as a worst-case strategy tailored for a specific device portfolio can often be adapted with minimal adjustments across different device lines. It is crucial to regularly monitor and re-evaluate worst-case scenarios, particularly following design modifications or manufacturing changes. Manufacturers must update their testing strategies accordingly to ensure continued compliance and maintain safety standards. Original Equipment Manufacturers have been leveraging advice and strategies from domain experts to reduce testing requirements by up to 85-93% for instruments and up to 60-68% for implants. This approach has led to strategy-driven decision-making and streamlined their submissions.
Key Benefits of Worst-Case Assessment
Conclusion
The worst-case approach is an invaluable tool for medical device manufacturers aiming to streamline biological testing processes and aligned with the proposed ISO 10993-1 and ISO 14971 expectation of risk-based approach. By focusing testing efforts on the most challenging device within a family, companies can achieve regulatory compliance, enhance efficiency, and accelerate time-to-market. Careful selection, thorough justification, and ongoing re-evaluation of worst-case scenarios are essential components of a successful biocompatibility strategy, ultimately supporting safer medical devices and better patient outcomes. Seeking guidance from experts with relevant experience at the inception of the testing strategy will help save time, money, and resources.







