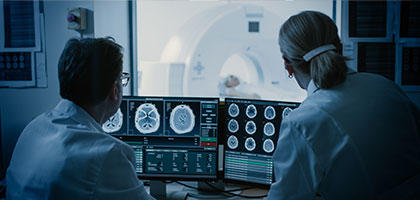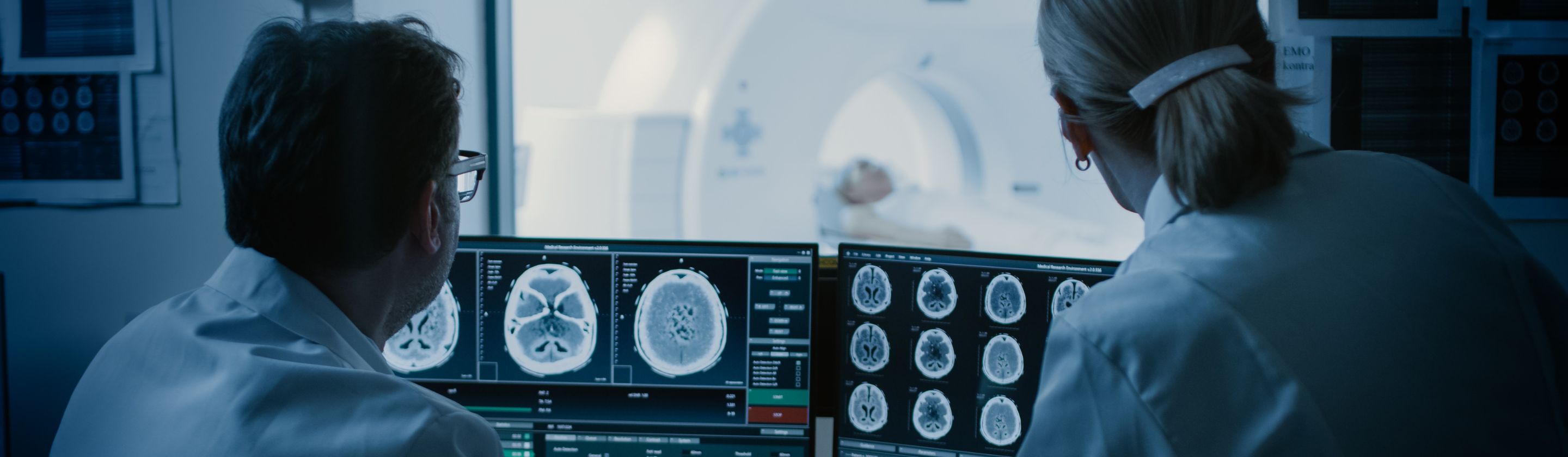
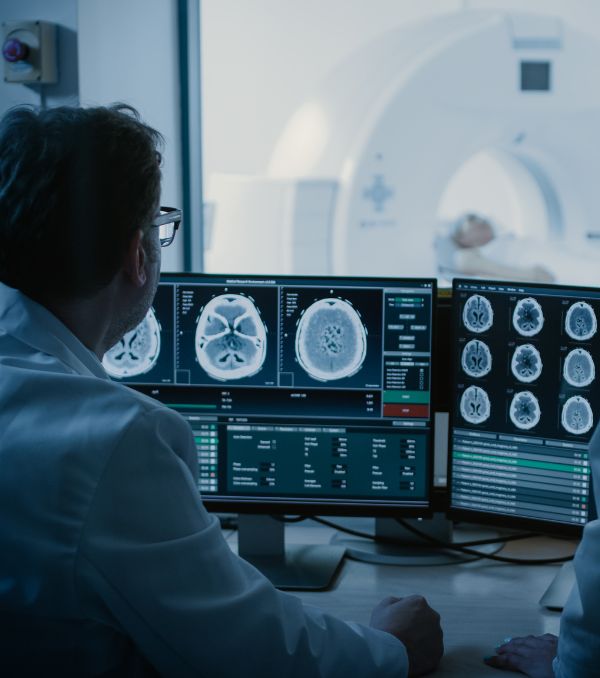
Tata Elxsi Launches a Global Technology Centre for Medical Devices from Bayer in Radiology
A centre to co-develop advanced radiology devices and technology that enable early and accurate diagnosis and treatment of critical illnesses.
Read more

Driving Critical Care Innovation with Dräger
Tata Elxsi and Dräger Establish an Innovative Partnership to Drive Critical Care Innovation in India
Read more

Building Next-Gen Medical Devices
In today’s rapidly evolving healthcare landscape, developing medical devices that seamlessly blend user-centric design, regulatory compliance, and technological innovation is paramount. At Tata Elxsi, our medical device engineering services help you navigate complex compliance pathways, expedite prototype development, and deliver robust, market-ready solutions. We combine clinical insights with AI-driven healthcare solutions to deliver superior products.
By leveraging industry-best practices, ISO standards, and wearable devices expertise, we ensure your devices meet global benchmarks while addressing evolving patient and physician needs. Whether creating a breakthrough concept or refining an existing product, our approach focuses on optimized outcomes across the entire product lifecycle.
Connected Care Solution


Here’s How We Help Medical Device OEMs
End-to-End Conceptualization and Design
- We conduct rigorous user research, clinical immersion, and concept ideation to shape intuitive, ergonomic medical device designs that enhance user experience.
- Our cross-functional teams ensure device architectures are future-proof, reducing iteration cycles and development costs.
Advanced Engineering and Validation
- We deploy cutting-edge simulation and verification methods to meet stringent regulatory requirements, including FDA guidelines.
- Our team applies data-driven insights and automated testing to guarantee consistent quality and patient safety. We also incorporate cybersecurity measures to protect sensitive patient data.
Lifecycle Management and Sustenance
- From product updates to post-market surveillance, we provide continuous support for evolving market demands.
- We optimize supply chain processes and implement sustainable manufacturing practices for cost-effective, eco-friendly device performance. This end-to-end approach ensures timely regulatory renewals, enhanced market presence, and lasting device relevance.
Medical Device Engineering Services Framework

The medical device engineering service framework provides a holistic approach to product development, starting from requirement gathering to production readiness. It integrates core engineering phases with digital enablers like connectivity, mobility, analytics, and cloud to support smart, connected medical devices. The framework also includes compliance checks, vendor collaboration, and production planning to ensure quality, speed, and regulatory alignment. This end-to-end model streamlines innovation, reduces development risks, and accelerates time-to-market for medical device manufacturers.
Early-Stage Development
The framework begins with requirement understanding, where clinical, technical, and regulatory needs are captured. This is followed by concept building, where user-centric ideas are translated into viable product strategies. The next phase, development, includes architecture, design, and engineering, aligning all components for functionality and compliance.
Digital Enablement & Prototyping
Core digital enablers such as connectivity, mobility, analytics, and cloud are embedded to support intelligent features, remote access, and scalable infrastructure. These technologies are key to modern medical devices. Once the core is in place, pilot building helps prototype and test solutions for usability, safety, and feedback-driven refinement.
Validation & Deployment
In the final stages, integration and testing verify system performance and ensure all components work seamlessly. Pre-compliance testing prepares the device for regulatory submission. The transfer to production step ensures design-to-manufacturing alignment, while vendor management secures a dependable supply chain, facilitating smooth scale-up and product launch.
Why Tata Elxsi?
- Deep healthcare domain knowledge for tailored solutions that meet strict global quality and compliance benchmarks.
- End-to-end development capabilities for efficient seamless integration, from ideation to deployment and complete post-market support.
- Proactive risk mitigation strategies with thorough validation, comprehensive regulatory foresight, and ongoing consistent compliance management.
- Cross-functional teams leveraging design thinking, engineering expertise, and robust data analytics for holistic product innovation.
- Commitment to sustainable practices, cutting-edge technologies, and patient-centric outcomes for significant lasting global market impact.
In Vitro Diagnostic Device for the Masses
In Vitro Diagnostic Device for the Masses

Hemex Health
"In our strategic mission to develop and launch a Lab-in-a-Box product, we are delighted to have the right partner in Tata Elxsi who understands the challenges of markets and possesses comprehensive capabilities from concept development to engineering and launch of medical products that meet regulatory standards."
Patti White, Chief Executive Officer
In Focus
Information Hub
-
What trends will shape the future of medical device engineering?
Emerging trends focus on miniaturization, connectivity, and patient engagement. Wearable and implantable devices are advancing rapidly, integrating real-time data monitoring and personalized treatment pathways. Cloud-based platforms enable seamless updates, remote diagnostics, and telehealth functionalities for improved patient care. Additionally, AI and predictive analytics will become more sophisticated, ushering in preventive healthcare and reducing hospital admissions. The push for sustainability is also driving the use of eco-friendly materials and energy-efficient designs. Finally, global regulatory environments are progressively accommodating innovative technologies, opening the door to faster approvals and broader market adoption.
-
What role does AI play in modern medical device engineering?
AI is transforming medical devices by enabling predictive maintenance, faster clinical decision support, and personalized healthcare solutions. Machine learning algorithms can interpret large volumes of patient data to detect patterns or anomalies that may otherwise remain hidden. This helps engineers refine designs, preempt failure modes, and optimize performance. AI-driven analytics also accelerate regulatory submissions by providing robust evidence of device safety and efficacy. As data collection and digital health tools expand, AI-powered features will become a standard component of cutting-edge medical devices, improving both outcomes and patient satisfaction.
-
How can manufacturers ensure usability and patient-centricity in medical device design?
Achieving patient-centricity starts with comprehensive user research and involving patients, clinicians, and caregivers in the design process. Human factors engineering helps identify potential design flaws, workflow inefficiencies, and safety risks before devices enter the market. Conducting iterative usability tests and simulations ensures that designs accommodate diverse user needs, including varying levels of technical proficiency. Additionally, incorporating feedback from pilot programs or small-scale trials refines the product further. By combining design thinking methods, real-world observations, and continuous user feedback, manufacturers can create devices that not only meet regulatory standards but also resonate with end-users’ expectations and comfort.
-
What factors affect time-to-market for new medical devices?
Time-to-market depends on multiple factors, including complexity of the device design, regulatory requirements, and availability of specialized resources. Prolonged prototyping phases, insufficient validation procedures, or late-stage design changes can introduce significant delays. Working with experienced engineering teams who have deep domain knowledge can expedite development by identifying potential pitfalls early. Additionally, digital collaboration tools, integrated supply chain strategies, and robust quality management systems help minimize bottlenecks. Streamlined documentation, prompt regulatory engagement, and pre-submission checklists also contribute to a shorter approval cycle. Ultimately, proactive planning and cross-functional coordination are key to accelerating time-to-market.
-
How is the regulatory landscape evolving for medical device engineering?
In recent years, global regulatory bodies, such as the FDA and European authorities, have tightened requirements to enhance patient safety and device efficacy. This includes updated guidelines for software integration, real-world evidence collection, and post-market surveillance. Additionally, new frameworks like the EU Medical Device Regulation (MDR) emphasize transparent documentation and continuous monitoring throughout a product’s lifecycle. To stay ahead, manufacturers must adopt proactive strategies like early regulatory engagement, robust quality management systems, and cross-functional alignment. By doing so, they can address compliance concerns more effectively and capitalize on emerging market opportunities.