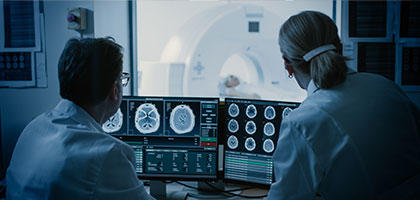
Charting the Future of Radiology: Innovation, Regulation, and Real-World Impact
Distinguish genuine innovation from hype, operationalize compliance for SaMD and AI/ML-enabled devices, and harness real‑world data (RWD) and post‑market evidence (RWE) to continuously improve safety, performance, and trust.
Watch Now
Tata Elxsi Launches a Global Technology Centre for Medical Devices from Bayer in Radiology
A centre to co-develop advanced radiology devices and technology that enable early and accurate diagnosis and treatment of critical illnesses.
Read more

Robotics and Automation Innovation Lab
Tata Elxsi partners with DENSO Robotics Europe and AAtek to drive the future of robotics automation in medical devices, pharmaceuticals, life sciences industries.
Read more

Driving Critical Care Innovation with Dräger
Tata Elxsi and Dräger Establish an Innovative Partnership to Drive Critical Care Innovation in India
Read moreA sustainable and safer future with differentiated medical devices & solutions
As the global healthcare landscape evolves toward value-based care, MedTech companies are compelled to develop high-performance, technologically advanced, yet affordable devices that address patient needs and regulatory standards. To thrive, organizations must adopt a holistic approach to medical device engineering, leveraging innovation and user-centric design. This ensures products are safe, effective, and comply with regulatory guidelines.
By focusing on novel R&D investments and cutting-edge robotics & automation, businesses can accelerate time-to-market, optimize resources, and improve clinical outcomes. Partner with Tata Elxsi to harness end-to-end services, from concept development to usability engineering, ensuring robust, patient-focused, and regulatory-compliant devices.
Ensuring Uninterrupted Market Presence with Proactive Obsolescence Management and Risk Mitigation
Business Challenge
Medical device companies grapple with rising development costs, rigorous regulations, and the need for continuous innovation to differentiate in a crowded market. Achieving faster product development while maintaining quality, safety, and affordability is critical. Additionally, aligning with emerging digital health trends demands seamless integration of data analytics, connectivity, and AI for real-time insights.
Medical Device Design and Engineering Service Offerings
Front-End Innovation in Medical Devices | Medical Device Engineering | Value Analysis & Value Engineering | Sustenance Engineering for Medical Devices | Intelligent Robotics and Automation
What's New
Information Hub
-
What are the emerging trends in medical device design for value-based care?
In value-based care models, medical device design increasingly focuses on personalization, connectivity, and data-driven insights. Devices integrate sensors, IoT capabilities, and AI algorithms to monitor patient vitals and deliver real-time feedback. Usability engineering ensures intuitive interfaces for diverse user groups, while robotics & automation streamline workflows in clinical settings. Additionally, regulatory bodies emphasize robust cybersecurity measures and evidence-based outcomes. Sustainability, through eco-friendly materials and efficient manufacturing, also shapes the future of MedTech innovation.
-
How can MedTech companies ensure compliance with evolving regulations in medical devices industry?
Staying ahead of continously evolving regulations requires proactive tracking of global standards, including FDA, EU MDR, and ISO guidelines. MedTech enterprises often partner with expert consultants to streamline documentation, risk assessments, and validation processes. Emphasizing usability engineering early in development helps meet human factors requirements, while adopting value analysis & value engineering can enhance both compliance and cost efficiency. Regular audits, robust quality management systems, and transparent supplier networks further minimize regulatory risks.
-
What role does robotics & automation play in advancing healthcare solutions?
Robotics & automation enhance precision, speed, and consistency in healthcare solutions, from surgical robotics to automated lab testing. They reduce manual errors, ensure repeatable processes, and free professionals for higher-value tasks. Automated systems also enable remote capabilities, supporting telehealth and decentralized testing. As AI-driven algorithms guide robotics, care delivery becomes more efficient, helping clinicians make evidence-based decisions. The result is improved patient safety, optimized resources, and faster diagnoses.
-
How do human factors & usability engineering impact medical device design?
By focusing on human factors & usability engineering, designers ensure medical devices are intuitive, safe, and accessible to diverse user profiles. This includes minimizing interface complexity, preventing operational errors, and enhancing user satisfaction. Rigorous testing under real-world conditions uncovers potential issues early, enabling timely improvements. Such user-centered development meets regulatory expectations, drives higher adoption rates, and ultimately elevates patient outcomes through devices that align with natural behaviors and clinical workflows.
-
What are key considerations in medical device innovation today?
Successful medical device innovation demands a convergence of human-centered design, regulatory foresight, and technology scalability. Companies must factor in real-world usability, clinical validation, cybersecurity, and compliance with evolving standards such as FDA, EU MDR, and ISO 13485. Partnering with the right medical device engineering and design teams ensures faster, safer, and more sustainable product launches.
-
Why is sustenance engineering critical for medical devices?
Sustenance engineering ensures that legacy and in-market medical devices remain compliant, secure, and supportable. It enables effective obsolescence management, value engineering, and faster adaptation to regulatory changes, extending product life and reducing total cost of ownership. As post-market requirements grow, a strong sustenance strategy is essential.