Charting the Future of Radiology: Innovation, Regulation, and Real-World Impact
Distinguish genuine innovation from hype, operationalize compliance for SaMD and AI/ML-enabled devices, and harness real‑world data (RWD) and post‑market evidence (RWE) to continuously improve safety, performance, and trust.
Watch Now
Tata Elxsi Launches a Global Technology Centre for Medical Devices from Bayer in Radiology
A centre to co-develop advanced radiology devices and technology that enable early and accurate diagnosis and treatment of critical illnesses.
Read more

Sustenance Engineering with Gen AI
Gen AI-powered sustenance engineering enables proactive risk management, optimized re‑engineering, and enhanced patient outcomes.
Watch Webinar

Robotics and Automation Innovation Lab in Frankfurt, Germany
A state-of-the-art facility aims to drive the future of robotics automation across sectors such as medical devices, pharmaceuticals, life sciences and food science.
Read PREmpowering MedTech OEMs to Simplify Sustenance & build competitive advantage
Healthcare industry today face shrinking margins, supply chain disruptions, and rapid tech shifts, all while managing regulatory pressures and sustainability goals. Component obsolescence, downtime, and rising warranty costs add further complexity to maintaining product continuity and reliability.
Tata Elxsi addresses these challenges through tailored sustenance engineering solutions, spanning obsolescence management, part harvesting, design optimization, and analytics-driven insights, enabling businesses to reduce costs, ensure compliance, and drive long-term product sustainability
Ensuring Uninterrupted Market Presence with Proactive Obsolescence Management and Risk Mitigation


Here's How We Help
Proactive Obsolescence and Change Management
- Extend product life through Last Time Buy (LTB) strategies, Fit-Form-Function (FFF) analysis, and robust change control processes.
- Leverage data-driven insights from service logs, field complaints, and root-cause analysis to drive design and process improvements.
Value Engineering and Compliance Excellence
- Enable alternate part and supplier qualification, process validation, and test method improvements for sustainable cost optimization.
- Strengthen regulatory compliance through supplier quality initiatives, proactive change management, and risk-based enhancements.
Reliability and Performance Engineering
- Elevate device reliability with Design for Excellence, security enhancements, and test fixture modernization.
- Ensure robust system-level V&V, covering security, portability, and interoperability for long-term patient safety.
TESS - Tata Elxsi Sustenance Service Framework
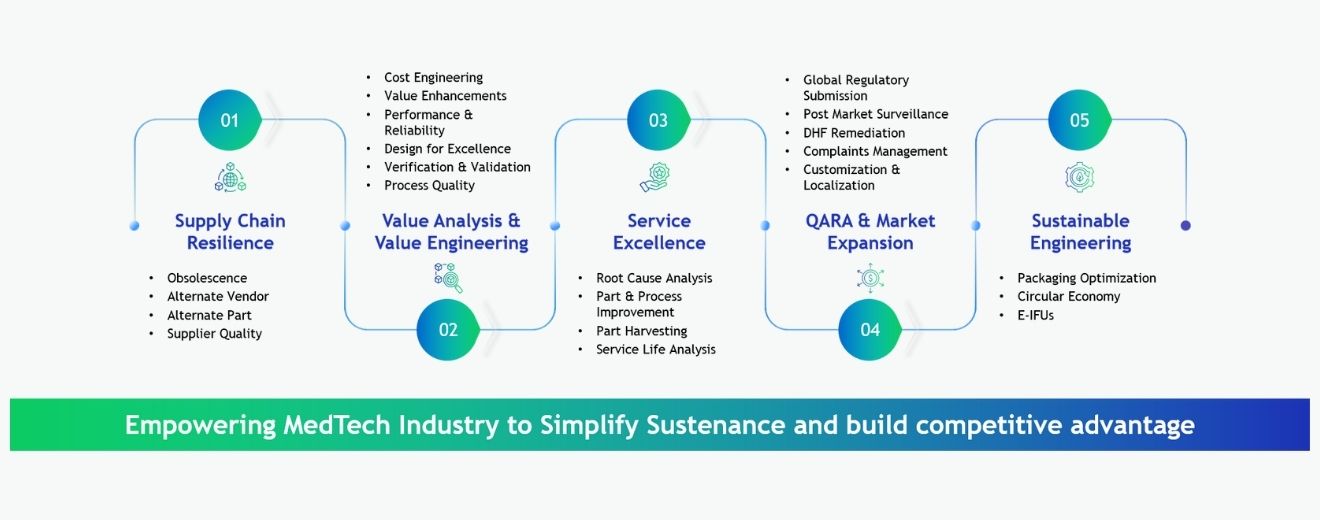
Our sustenance engineering framework unifies lifecycle management, regulatory continuity, and cross‑functional engineering expertise in a cohesive architecture. It connects end‑to‑end functions, from supply‑chain stability to post‑market quality, with scalable, domain‑specific modules for proactive risk mitigation, documentation accuracy, and global compliance. By centralizing engineering data and integrating performance insights, it streamlines change management, accelerates remediation, and preserves product reliability throughout the lifecycle.
Strategic Resource Models
Tap into a shared pool of 300+ sustenance engineers with cross-functional expertise across 7+ healthcare segments. Our flexible engagement models, ranging from managed services to staff augmentation—help reduce costs during low-demand periods and ensure business continuity with scalable, domain-specific support.
Obsolescence and Value Engineering
Mitigate supply chain risks and ensure part availability through proactive obsolescence management. We combine revenue assurance strategies with value engineering practices to improve BOM productivity, maintain compliance, and keep your products competitive throughout their lifecycle.
Regulatory-Aligned Sustenance Excellence
Leverage deep regulatory knowledge, accredited prototyping and test labs, and seamless supplier networks to manage recalls, CAPAs, and post-market surveillance. Our KPI-driven sustenance model ensures full traceability, efficient documentation, and global readiness across engineering, quality, and manufacturing.
Why Tata Elxsi?
- A global team of 300+ sustenance engineers with expertise in innovation, design, and engineering, advancing medical device development across 7+ healthcare segments, including homecare, diagnostics, therapeutics, and imaging.
- Flexible engagement models tailored to customer needs, reducing costs during low-demand periods and enhancing efficiency through shared resources and specialized skills.
- Prototyping and testing facilities supported by a network of manufacturers, suppliers, and accredited labs ensure seamless product development and sustenance.
- Extensive knowledge of regulatory landscapes and best practices enables precise implementation of support strategies and compliance for medical devices.
- Proactive obsolescence management secures revenue, sharpens strategic focus, and ensures a competitive edge.
Information Hub
-
How can sustenance engineering strategies help medical device manufacturers extend product lifecycle while ensuring compliance?
Sustenance engineering combines proactive maintenance, risk-based upgrades, and change management to keep devices performing reliably beyond their intended service life. By addressing regulatory updates, field feedback, and part obsolescence early, manufacturers can maximize their device investments while ensuring patient safety and ongoing compliance.
-
Why is proactive obsolescence management essential in today’s global medical device supply chain?
Component shortages and supply chain disruptions are increasingly common, putting device continuity at risk. Proactive obsolescence management helps manufacturers identify vulnerable components, qualify alternates, and maintain validated supply chains, ensuring devices remain available, safe, and regulatory-compliant even during global crises.
-
How do value analysis and value engineering (VAVE) contribute to the cost-effectiveness of medical devices?
VAVE systematically reviews designs, materials, and suppliers to find opportunities for cost reduction without compromising device quality or regulatory requirements. By qualifying alternative parts, optimizing processes, and reducing material waste, manufacturers can sustain profitability while maintaining clinical performance.
-
What challenges do MedTech companies face in maintaining medical device safety over a 10–15 year product lifecycle?
Over such long lifecycles, medical devices must adapt to changing standards, cybersecurity threats, new patient expectations, and environmental conditions. Sustenance engineering helps manage these challenges through structured updates, robust testing, and documented design controls to maintain reliability and patient safety.
-
How does partnering with an engineering services provider support successful medical device sustenance programs?
Collaborating with a specialized partner gives manufacturers access to skilled resources, advanced tools, and proven methodologies for sustaining products. From managing DHF updates and post-market surveillance to handling supplier qualifications and continuous improvements, a strong partner reduces lifecycle costs while protecting the device’s market value.











